Chlorosomes
Overview |
|
Chlorosomes are light-harvesting complexes found in photosynthetic bacteria belonging to three diverse phyla: Chlorobi, Chloroflexi and Acidobacteria. A unique property of chlorosomes among all light harvesting complexes is that their main pigments, bacteriochlorophylls (BChl) c, d or e, are organized in the form of an aggregate without forming complexes with proteins that maintain the distances and mutual orientations between pigments in other photosynthetic complexes. The aggregates inside a chlorosome are composed of many thousands of tightly associated BChl molecules making the chlorosome the largest light-harvesting complex known to date. The aggregation also leads to strong excitonic coupling between the BChls which provides the basis for high light-harvesting efficiency. Consequently, some of the chlorosome-possessing bacteria can grow under extremely low light conditions and chlorosomes are considered the most efficient antennae found in nature. The self-assembling aggregates of chlorosome pigments have potential use in nanotechnologies, e.g. artificial photosynthesis or molecular electronics. The chlorosome structure is still a matter of debate. In the past, models in which BChl pigments were organized into hexagonally packed rods (diameter 5 or 10 nm) were proposed on the basis of freeze fracture electron microscopy and spectroscopic constraints. However, recent higher resolution electron cryomicroscopy images and X-ray diffraction results revealed that the pigments form a lamellar phase. Some of the results concerning general features of the chlorosome, including structure, energy transfer and self-assembly of BChl aggregates, which were obtained with involvement of our group, are described below. More detailed information about the structure, function and assembly of the chlorosome can be found in our recent review. |
|
1. Structure of the chlorosome |
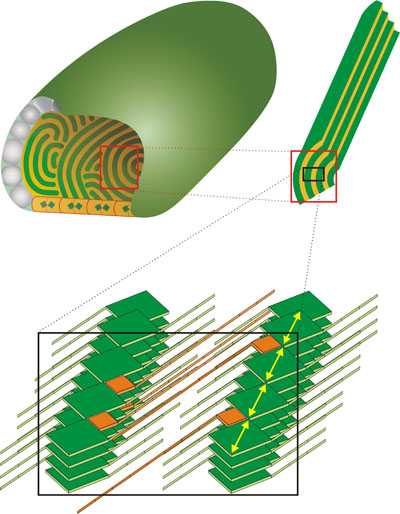
Cryoelectron microscopy (cryo-EM) images revealed a striation pattern often parallel to the long axis of the chlorosome with spacing ranging from ~2 nm for Cba. tepidum (see the next paragraph or Psencik et al. 2004) (cryo-EM images of chlorosomes (Fig. 1) for download in higher resolution, 3.3 MB to 2.6-4.0 nm for Cfl. aurantiacus (Psencik et al. 2009). Corresponding solution X-ray scattering, which reports average structure of all the chlorosomes in the sample, exhibited features with comparable spacings (from 2.1 nm for Cba. tepidum to 3.3. nm for Cfl. aurantiacus) and a scattering pattern typical of the lamellar phase seen for other amphipathic self-assembling molecules (lipids, block copolymers etc.). The lamellar arrangement has been so far observed in green- and brown-colored Chlorobi species and Cfl. aurantiacus suggesting that this is a universal feature of chlorosome structure. From cryo-EM observation of the same chlorosome from different projection angles (tilt series) it became evident that the lamellar system cannot be planar but must be curved (Psencik et al.2004). a) general features of the lamellar model Schematic diagram on the right shows the shape and interior of a typical chlorosome. The baseplate at the bottom of the chlorosome is made of CsmA protein dimers, each containing one BChl a molecule (blue-green rectangle), the orange background of the baseplate alludes to the presence of carotenoids in the baseplate. The envelope is formed mainly by proteins (the gray particles) while lipids fill the space between the proteins (cyan).The curved lamellar layers (green) inside the chlorosome consist of BChl aggregates. Orientation of the Qy transition dipole moments of BChl molecules is shown by yellow arrows in the enlargement. The esterifying alcohols of BChls extend to both sides of the layer and facilitate formation of lamellar structures by the hydrophobic interaction between the esterifying alcohols of BChls neighboring layers. The hydrophobic space formed by interdigitated esterifying alcohols (orange) is also occupied by other important constituents of chlorosomes: carotenoids and quinones (Psencik et al. 2006). A close proximity between carotenoids and BChls is required for both 1) photoprotection of BChls by triplet-triplet quenching and 2) energy transfer from the short lived S2 state of carotenoids. In other light harvesting complexes this is achieved via association with a protein scaffold, which is absent from the chlorosome interior. In chlorosomes, the required close-range association is likely to be achieved by π-π interactions of the conjugated systems of BChls and carotenoids (Fuciman et al. 2011). b) different forms of lamellar arrangement |
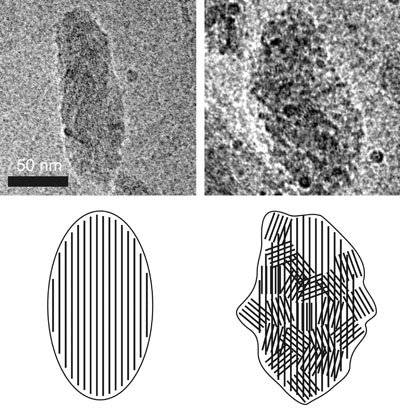
Chlorobi can be divided to green-colored bacteria containing BChl c or d and brown-colored bacteria with BChl e. Cryo-EM images on the right illustrate the differences in the shape and internal organization of chlorosomes from both groups. The chlorosomes from green-colored bacteria are usually smooth (as the chlorosome from Cba. tepidum on the left side of the figure, Psencik et al. 2004) and the chlorosomes from brown-colored bacteria often exhibit a rough surfaces (as the chlorosome from Chl. phaeovibrioides on the right side of the figure, Psencik et al. 2006). The rough surface is due to organization of the lamellar system into domains with different orientation, as illustrated on the schematic representation below the cryo-EM images. The lamellar system in chlorosomes from green-colored Chl. tepidum is usually parallel to the long axis of the chlorosome (the scheme on the left). As the transition dipole moments of the BChls are oriented along the lamella, such an arrangement would be very efficient in absorbing only of the light with a polarization component along the long axis of the chlorosome. The brown bacteria often live at extremely low light conditions and they need to utilize any available photon. In contrast to green-colored bacteria, the chlorosomes from the brown-colored species often contain domains of lamellar aggregates with almost random orientation (the scheme on the right). The existence of domains may be essential for survival because they provide a transition dipole component in the perpendicular direction and the chlorosome can efficiently absorb photons of any polarization (Psencik et al. 2006). |
|
2. Self-assembly of chlorosomal pigments |

Aggregates with very similar optical properties to those of chlorosomes can be prepared also artificially, either in non-polar solvents, or in the presence of lipids in aqueous buffer. We have observed that also chlorosomal carotenoids and quinones promote BChl c assembly in aqueous solutions in vitro where BChl c alone remains partly soluble (Klinger et al. 2004, Alster et al. 2008). In addition, it has been shown that the esterifying alcohols (in addition to the presence of either lipids, carotenoids or quinones) are necessary for the formation of the aggregates in an aqueous environment, but not in a non-polar solvent such as hexane (Zupcanova et al. 2008). The results indicate that the hydrophobic interactions are important for assembly of BChl molecules in aqueous solutions |
|
3. Excitation energy transfer in chlorosomes |

Simplified representation of the main transitions and energy transfer steps in chlorosomes is shown on the right. Horizontal lines represent states with a high (solid line) and low (dashed) oscillator strength (including forbidden states). Vertical arrows represent absorption (blue), fluorescence (red) and internal conversion (black). Oblique arrows represent exciton relaxation (black) and energy transfer (green). Absorption (blue) and fluorescence (red) spectra of BChl e containing bacterium Chl. phaeobacteroides are shown on the right for comparison. Note that the fluorescence spectrum is dominated by the emission from BChl a, (maximum at ~810 nm) despite the fact that these chlorosomes contain only about ~1% fraction of BChl a. This is due to the efficient energy transfer from BChl e aggregates to the baseplate. Energy absorbed by the Soret band of aggregated BChl (~425-525 nm for BChl e) and carotenoids (Car, ~425-525 nm for isorenieratene) is transferred to the Qy band of aggregated BChl (~680-750 nm for BChl e). From the Qy band of the aggregated BChl (emission around ~740 nm for BChl e), the excitation energy is transferred to BChl a in the baseplate of the chlorosome (emission around ~810 nm) and further towards the reaction centres in the cytoplasmic membrane. In the following, particular excitation energy transfer steps are described, as revealed for BChl e and isorenieratene containing bacterium Chlorobium phaeobacteroides by femtosecond spectroscopy: |
|
a) energy transfer from Car to BChl 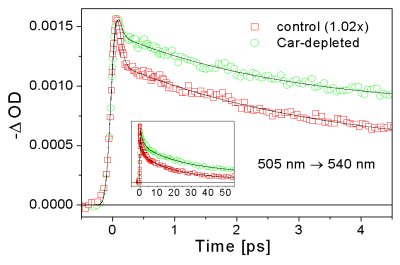
The fastest energy transfer process observed in chlorosomes is the excitation transfer from the S2 state of Car to BChls. This process is characterized by a rate constant of (65-100 fs)-1 and efficiency of 60-70%. This efficiency is rather high, taking into account very short intrinsic lifetime of the S2 state of carotenoids (~150 fs), which is shortened to ~50 fs by the energy transfer. The dark S1 state, characterized by a relatively long lifetime (~10 ps) is not involved in the excitation energy transfer, which is the result of the S1 state being dipole forbidden. The internal relaxation from the Soret to Qy band of BChl e occur also with a <100 fs lifetime. - details can be found in Psencik et al. (2002) Photosynth. Res. 71 |
|
b) energy transfer within BChl aggregates 
The Qy band of BChl e consists of a number of so-called exciton levels arising from a monomeric energy level being split by dipole-dipole interactions of densely packed pigments forming the aggregate. This band can be populated by energy transfer from Car, internal relaxation from the Soret band or by direct excitation. Study of the energy transfer within the Qy manifold is complicated by strong singlet-singlet excitation annihilation, which is a consequence of high density of pigments. In order to reach annihilation-free conditions, pump intensities in the order of 1011 photons×pulse-1×cm-2 are required. The most important consequence of applied very low excitation doses was a first direct observation of a subpicosecond exciton relaxation within the BChl e manifold at room temperature, manifesting itself as a rise in the red part of the Qy absorption band of the BChl e aggregates. - details can be found in Psencik et al. (2003) Biophys J 84 |
|
c) subsequent energy transfer processes 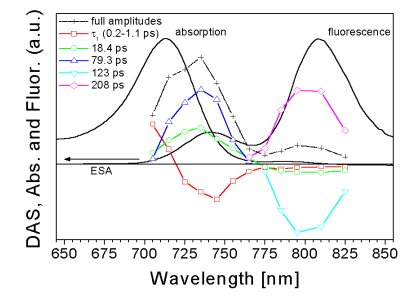
The fast exciton relaxation is connected with the localization of the energy and Förster hopping between the localized sites governs further energy transfer steps. The subsequent decay of the kinetics measured in the BChl e region and the corresponding rise in the baseplate BChl a is not single-exponential and at least two components are necessary to fit the data, corresponding to several BChl e to BChl a transfer steps (~1 ps, ~20 ps, ~100 ps). Under annihilation-free conditions, the anisotropy kinetics show a generally slow decay within the BChl e band (10-20 ps) while it decays more rapidly in the BChl e region (~1 ps). Analysis of the experimental data gives a detailed picture of the overall time evolution of the energy relaxation and energy transfer processes within the chlorosome. - details can be found in Psencik et al. (2003) Biophys J 84 |
